Why is the cleaning of implants prior to coating a critical step in the manufacturing process?
Prior to coating, implants must be thoroughly cleaned of any contaminants such as oils, greases, machining fluid residues, or particles that can interfere with coating adhesion and lead to uneven coatings. And improving surface adhesion also plays an important role in durability. Effective cleaning helps to create a clean and uniform surface to which the coating can better adhere. This is essential for the long-term stability and functionality of the implant..
Thorough cleaning eliminates potential sources of contamination that may occur during the manufacturing process This is especially important to ensure implant sterility and prevent patient infection.
"The MDR requires clinical evaluation of medical devices, such as implants, to confirm their safety and performance. The effectiveness of cleaning methods and procedures may be part of this clinical evaluation to ensure that potential risks associated with cleaning are adequately addressed", Fabio Cordaro.
What about compliance with regulations and standards?
When it comes to "cleaning, passivation and final packaging," medical device manufacturers need to focus on the FDA (Food and Drug Administration) in America or the MDR (Medical Device Regulation) in the EU, depending on the country. Here are some of the reasons compliance is so important to the medical device industry:
- Proper parts cleaning procedures, passivation processes, and sterile packaging are critical to avoid contamination and ensure product integrity throughout its lifecycle - protecting patient health. These regulations ensure high quality standards in the manufacturing process.
- Regulations require the documentation and traceability of materials and processes used in the manufacture of medical devices. This helps manufacturers to identify problems that may occur during the manufacturing process. In other words, always know exactly what, where and when.
- Compliance with FDA and MDR regulations is often a prerequisite for market access, not only in the United States and the European Union, but also in many other countries around the world. As MedTech manufacturers go global, they must comply with these regulations and need a supplier that can help them navigate the regulatory landscape, rather than just selling equipment detached from the overall process.
Ecoclean has developed a passivation process called Pulsated Pressure Passivation (PPP) to meet the specific needs of the medical industry. How does it work and what are the benefits for MedTech manufacturers?
As far as parts cleaning is concerned, we already work with a process called PPC (Pulsated Pressure Cleaning), which consists of reducing and increasing the pressure in the process chamber filled with the cleaning medium to reach the areas to be cleaned or rinsed, taking advantage of the air contraction once the chamber is ventilated. We have used our experience in this process to offer a technology for the passivation process that we call PPP (Pulsated Pressure Passivation), which allows the surface to be treated with a protective layer regardless of the geometry of the component, since this change in pressure inside the process chamber causes the passivation to chemically flow into all the cavities and holes.
The new passivation process from SBS Ecoclean Group is a quantum leap for medical and high-end technologies.
Traditional atmospheric methods only clean and passivate the external geometry of the parts.
With the new process we are able to clean and passivate implants and instruments even in capillaries, internal geometries and blind holes.
We would be happy to invite you to our application laboratory to demonstrate this groundbreaking process.
PPC and PPP are exclusive cleaning and passivation processes developed by Ecoclean!
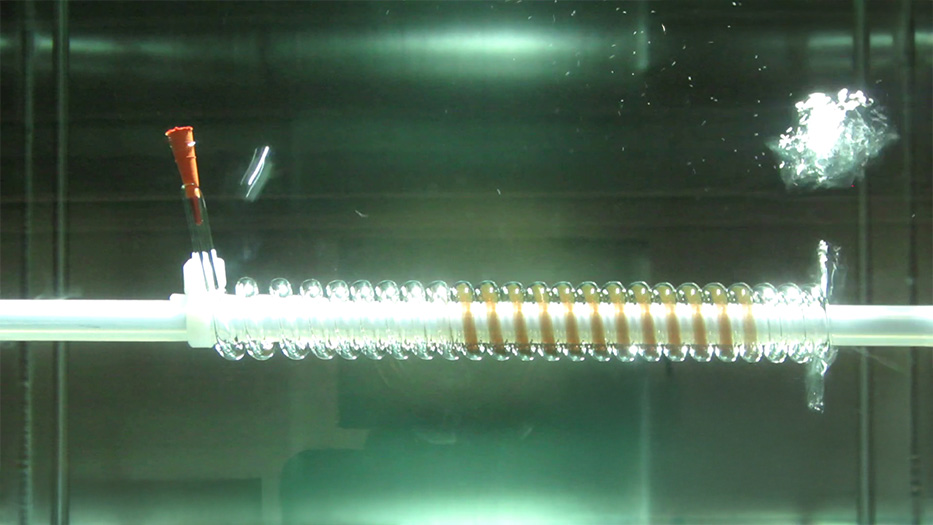
PPP (Pulsated Pressure Passivation) allows the surface to be treated with a protective layer regardless of the geometry of the component, since the pressure change within the process chamber causes the passivation to chemically flow into all cavities and holes.
We have a MedTech Test Center at our Ecoclean Center of Competence in Monschau, Germany. What do we offer there?
Ecoclean has a fully equipped Technology Center in Monschau, Germany, with a cleanroom laboratory and a validated cleanroom for system and process design. This facility allows us to precisely develop customized solutions for various medical device applications. In addition, our solutions can be easily duplicated for implementation across multiple manufacturing sites.
The MedTech Test Center offers a range of solutions for cleaning and packaging medical implants and instruments. Manufacturers will find our complete portfolio, from single-chamber solvent and water-based systems to multi-stage ultrasonic cleaning machines. With this infrastructure, we can evaluate the best process for MedTech needs. It is important to remember that one of the biggest challenges for manufacturers in the medical device component industry is keeping up with the ever-changing regulatory environment. This is where the SBS Ecoclean Group comes in, offering not only the technology of our systems for parts cleaning, passivation and sterile packaging, but also qualified support to meet the requirements and a recalibration service to bring production up to date. And, of course, always in compliance with the latest MDR and FDA regulations.

Which Ecoclean parts cleaning system for the MedTech industry combines innovative technology, sustainability and precision?
The SBS Ecoclean Group brands Ecoclean and UCM AG are collaborating on advanced cleaning systems for medical devices, particularly in the field of endoscopy. The result of this collaboration is the UCMPerformanceLine, an ultrasonic multi-chamber immersion cleaning system designed to maintain the high standards of cleanliness required for optical lens systems in medical endoscopes. The UCMPerformanceLine is engineered to efficiently and accurately clean small, delicate parts such as coated lens systems, ensuring they meet stringent cleanliness specifications. The system features a modular design for customized cleaning processes, automated part transport, and is directly connected to a cleanroom.
What else do we have in our portfolio of medical component cleaning systems?
We combine Ecoclean and UCM AG technologies to provide solutions from A to Z, depending on customer requirements and after conducting trials at our test centers around the world. For pre-cleaning of MedTech parts and components, we have aqueous or solvent-based chamber systems such as the EcoCwave or the EcoCore. For parts cleaning after finishing, we offer UCM inline immersion systems and finally for final cleaning and/or passivation we offer among others the UCM PerformanceLine. For very small and complex parts we have for example the UCMIndexLine - in summary: we have the right system for the required application.
What technologies distinguish Ecoclean's MedTech solutions?
Ecoclean differentiates itself through the use of specialized medical device software, RFID technology, and meticulous audit trails. These technologies ensure that all component identification, documentation and traceability requirements are not only met, but exceeded.
Our team of experts is dedicated to providing comprehensive support throughout the qualification process, including IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification) upon request. We understand the importance of compliance and are committed to guiding our customers every step of the way.









